The Zuo Labat the School of Physical Science and Technology recently published details oftheir breakthrough method in the journal Angewandte Chemie-InternationalEdition, titled “Photocatalytic C–C Bond Cleavage and Amination ofCycloalkanols by Cerium(III) Chloride Complex”. Dr. Guo Jingjing and Dr. Hu Anhuaare co-first authors and Professor Zuo was the corresponding author.
Over the last60 years, tremendous progress has been made in the area of organic synthesis,which has become the most transformative power in drug discovery. Although it’ssaid that nowadays, given enough time and resources, any desired molecule can besynthesized, to achieve that goal in a more efficient and economical pathway isstill an unmet need in the pharmaceutical industry. The direct functionalizationstrategy of ubiquitous C–H and C–C bonds has been proven to be a promising solutionto this challenge, and therefore has attracted significant research efforts.Due to the inertness of those bonds, precious transition metals and harshconditions normally have to be employed to achieve effective transformations. Developingmore sustainable catalysis system and affordable catalysts to cleavage andfunctionalize C–H and C–C bonds has become the research focus of the Zuo lab.
Over thelast five years, photoredox catalysis has made tremendous progress towardsselective C–H bond functionalizations. Nevertheless, regarding more challengingC–C bonds, breakthroughs have only been made recently with restrictedsubstrates scope. Taking advantage of the luminescence properties of Ce(III)compounds and the highly oxidizing nature of Ce(IV) species, the Zuo lab hasdeveloped a general Ce(III) chloride-based photo catalyst system for theselective C–C bond cleavage/amination of unstrained cycloalkanols. A broadarray of alcohols, including complex terpenols and sterols, have been demonstratedto undergo selective C–C bond scission/amination under very mild conditions. Thismethod represents the first photocatalytic C–C bond cleavage andfunctionalization of secondary cycloalkanols. This operationally simplephotoredox reaction utilizes easily accessible ingredients: commercial blueLEDs, the catalytic combination of CeCl3(an inexpensive, commonlyused Lewis acid) and TBACl (a commonly used phase transfer catalyst).
This Ce(III)chloride-based photo catalyst represents a novel scaffold for photoredoxcatalysis. With its unique metal-centered photoexcitation and the broadoxidation-reduction potential scope, they are confident that moretransformations taking advantage of this scaffold can be realized. The ease ofsimply mixing bench chemicals in situ, with no need for tedious synthesis ofluminescent transition-metal complexes, will certainly be embraced by morepractitioners of organic chemistry in the future development of additionalphotoredox reactions.
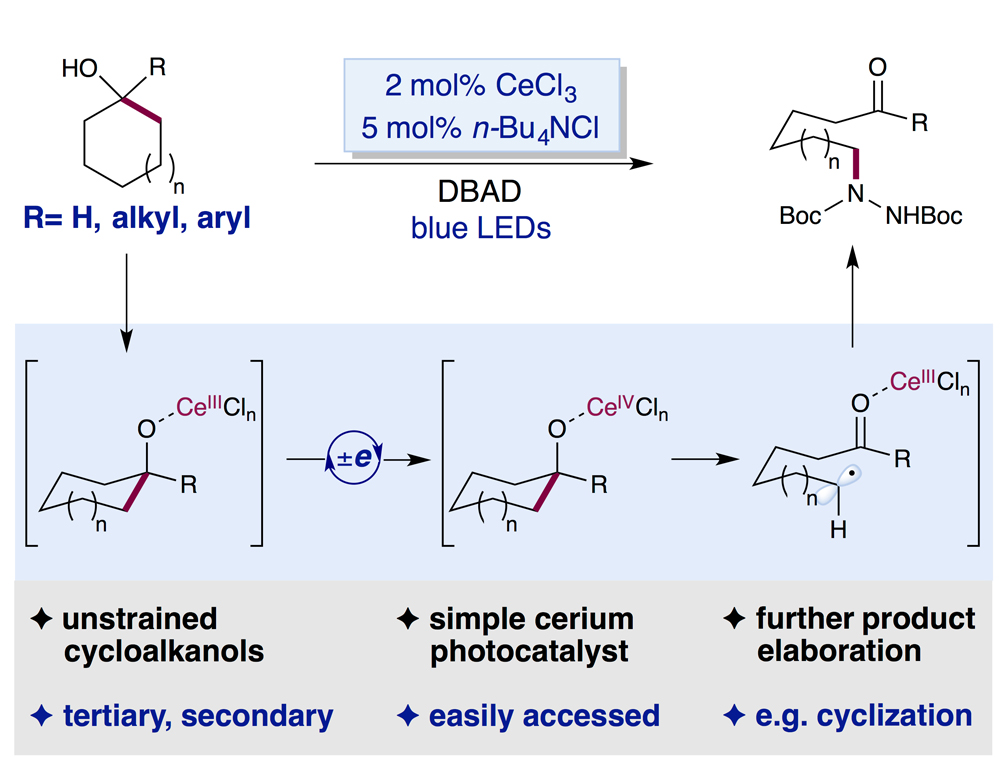
Figure 1 C–Cbond cleavage and amination via Ce(III) photo catalyst

Figure 2 Dr.Guo JingJing (right) and Dr. Hu Anhua (left) in lab

