Researchers from the School of Physical Science and Technology, ShanghaiTech University has revealed a new way to synthesize allyl- and benzyl-substituted quinone compounds. The results are summarized by graduate student Xiao-Long Xu and Assistant Professor Zhi Li of SPST in a communication entitled “Catalytic Electrophilic Alkylation of p-Quinones via a Redox Chain Reaction”, which is published online in Angewandte Chemie International Edition on May 22, 2017.
Quinone derivatives are a class of organic compounds with unique structures and functions. Functionalized quinones such as Coenzyme Q10 and Vitamin K are key electron transporters in cellular respiration process and photosynthesis process, respectively. Many drugs also contain quinone functional group, such as Idebenone which may alleviate Alzheimer’s disease, and Seratrodast which is an anti-asthma drug. Thus, efficient, cost-effective and green synthetic methods of quinone derivatives are highly desirable.
Quinone derivatives are usually obtained from the oxidation of corresponding functionalized hydroquinones, phenols or aryl ethers. These methods usually require large amount of catalysts, protecting reagents and oxidants. On the other hand, this new method converts simple quinones and allylic/benzylic esters into functionalized quinones under mild conditions, using only very low loading of Lewis acid Hf(OTf)4 as the catalyst.
In addition, the mechanism of this new method is also a quite interesting puzzle. Both reagents, i.e. the quinone and the ester, are common electrophiles, which in principle do not react with each other. After careful studies, we hypothesize that the reaction undergoes a “redox chain mechanism”. The rate-determining step is a Lewis acid-catalyzed electrophilic substitution of hydroquinone with ester, generating alkylated hydroquinone. Then a fast equilibrium between the alkylated hydroquinone and remaining quinone generates the final alkylated quinone product, while replenishing hydroquinone for the previous step. The initial hydroquinone is either directly added or reduced in situ from the quinone. This mechanism resembles a radical chain reaction consisting initiation, propagation and termination stages. Kinetics studies and various control experiments demonstrated the preliminary feasibility of such a mechanism. Catalytic amount of Hantzsch ester can be used as the universal initiator for different quinone reagents.
This work demonstrates new ways and concepts for quinone synthesis, as well as preliminary mechanistic studies. It is supported by National Natural Science Foundation of China and ShanghaiTech University start-up funding.
Read more at: http://onlinelibrary.wiley.com/resolve/doi?DOI=10.1002%2Fanie.201702885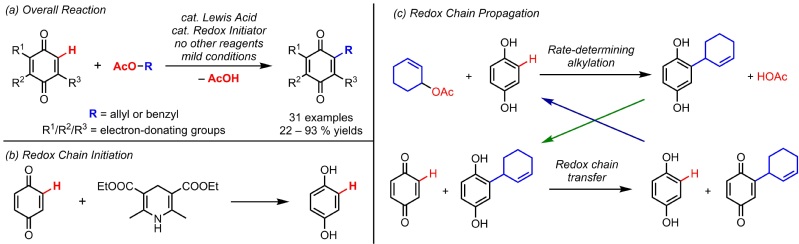
(a) Redox Chain Reaction (b) Redox Chain Initiation (c) Redox Chain Propagation
- About
- News
- Faculty
- Research
-
Academics
- School of Physical Science and Technology (SPST)
- School of Life Science and Technology (SLST)
- School of Information Science and Technology (SIST)
- School of Entrepreneurship and Management (SEM)
- School of Creativity and Art (SCA)
- Institute of Humanities (IH)
- School of Biomedical Engineering (BME)
- Shanghai Institute for Advanced Immunochemical Studies (SIAIS)
- iHuman Institute
- Institute of Mathematical Sciences (IMS)
- Center for Transformative Science (CTS)
- Institute of Carbon Neutrality (ICN)
- Shanghai Clinical Research and Trial Center
- Tech Transfer
- Global
- Campus Life

