Autophagy is a conserved eukaryotic cellular process that delivers protein aggregates, damaged organelles, and invaded pathogens to the lysosome for degradation. Reticulophagy is a specialized form of autophagy that selectively breaks down damaged or redundant endoplasmic reticulum (ER) using the autophagy-lysosome pathway, thus maintaining ER homeostasis and dynamic equilibrium. Reticulophagy receptors interact with the autophagy core protein, one of the LC3 family members, targeting specific ER regions for lysosomal degradation. Recent research has highlighted the significance of post-translational modifications in regulating the autophagy pathway. However, the precise involvement and regulatory mechanisms of ubiquitination in reticulophagy remain incompletely understood.
Associate Professor Liu Yanfen’s group in the School of Life Science and Technology (SLST) has been long engaged in the research on the mechanisms of ubiquitination regulation of autophagy and the selective autophagy of the ER, mitochondria, and stress granules. In their recent study, the researchers unveiled a novel regulatory mechanism of ubiquitination in the selective autophagic degradation of the endoplasmic reticulum (ER). The groundbreaking study titled “USP20 deubiquitinates and stabilizes the reticulophagy receptor RETREG1/FAM134B to drive reticulophagy” was published in the journal Autophagy on May 5.

In this study, the researchers conducted a comprehensive screening of the deubiquitinating enzymes (DUBs) family members and identified USP20 as a regulator of the autophagy pathway under starvation conditions. Despite lacking a transmembrane domain, USP20 is recruited to the ER by interacting with the ER membrane proteins VAPA/B.
The researchers further explored the regulatory role of USP20 in reticulophagy. By combining cellular biology, biochemical, and high-resolution imaging techniques, they discovered that USP20 deubiquitinates and stabilizes RETREG1, preventing its degradation via the ubiquitin-proteasome pathway. High-resolution imaging revealed an intercalated localization of USP20 and RETREG1 on the ER, with increased overlapping signals during starvation treatment. Through the deubiquitination regulation of RETREG1, USP20 facilitates its interaction with the autophagy core protein LC3, thereby inducing reticulophagy. Additionally, USP20 enhances the interaction between RETREG1 and VAPA/B, resulting in the recruitment of autophagy initiation complex proteins to specific ER regions and further promoting reticulophagy (Figure 1).
This study elucidates the significant regulatory mechanisms of ubiquitination in reticulophagy, providing new insights into the understanding of selective autophagy. Diseases associated with the ER, such as neurodegenerative diseases, are closely related to impaired reticulophagy. Therefore, these findings offer new targets and strategies for the treatment and prevention of related diseases.
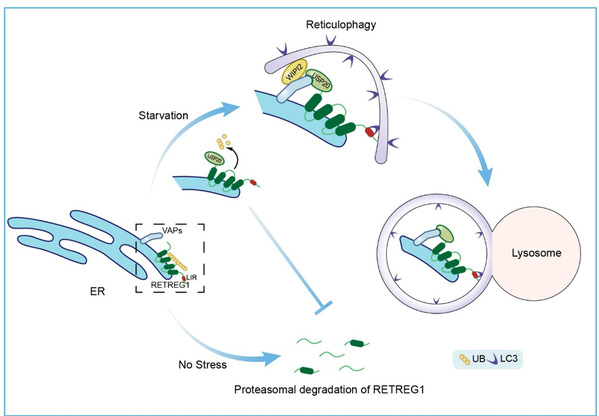
Figure 1: USP20 regulates the deubiquitination of RETREG1 and promotes reticulophagy.
USP20 is recruited to the ER by the ER membrane proteins VAPA/B. USP20 deubiquitinates and stabilizes RETREG1, protecting it from degradation via the ubiquitin-proteasome pathway. Under starvation conditions, USP20 and RETREG1 colocalize in specific regions of the ER, and USP20 further recruits VAPA/B to these foci. This concerted action leads to the recruitment of early autophagy proteins, including WIPI2, to the specific regions of the ER, further promoting reticulophagy.
SLST PhD candidate Zhang Man is the first author of the paper, Prof. Liu Yanfen is the corresponding author. ShanghaiTech University is the primary affiliation.
*This article is provided by Zhang Man

