Over the past few years, the widespread application of brain organoid models in research has been accompanied by advancements in the methods to construct brain organoids, allowing for more accurate modeling of the human brain in a dish. Common strategies for building brain organoids include unguided and guided differentiation, each serving a crucial role depending on the specific research context. The human brain is an intricate and complex system, and developing organoid models targeting particular brain subregions and nuclei remains a significant challenge. While several brain region-specific organoids have been successfully developed in recent years, studies focused on constructing brain organoids that mimic the features of specific brain nuclei in vitro are still limited. On August 28, 2024, the Xiang Yangfei Lab at the School of Life Science and Technology (SLST) of ShanghaiTech University published a paper titled “Generation of human region-specific brain organoids with medullary spinal trigeminal nuclei” in the journal Cell Stem Cell, reporting a new method for constructing brain organoids.
The medulla oblongata plays a crucial role in regulating fundamental life functions. However, there is still a lack of brain organoid models targeting this area, particularly in constructing nucleus-specific features within the medulla. In this report, researchers have focused on the spinal trigeminal nucleus (SpV) in the medulla. The SpV is the largest cranial nerve nucleus and an essential component of the trigeminal nucleus. As an important sensory relay structure in the brain, the SpV is primarily responsible for relaying sensory information from the craniofacial region to the thalamus. Although the SpV is closely involved in maintaining normal physiological functions and the occurrence of various pathological mechanisms, there is currently no existing model for the human SpV nucleus.
To establish human SpV organoids, this study investigated the guided 3D differentiation of human pluripotent stem cells. By comparing these organoids to other brain region-specific organoids, such as previously established cortical and thalamic organoids, as well as in vivo brain tissues, researchers characterized the identity of SpV organoids and delineated lineage specifications during organoid development. Additionally, this study examined the structural and functional characteristics of long-term cultured SpV organoids. SpV neurons formed self-organized axonal bundle structures during long-term culture, indicating their potential projection capabilities.
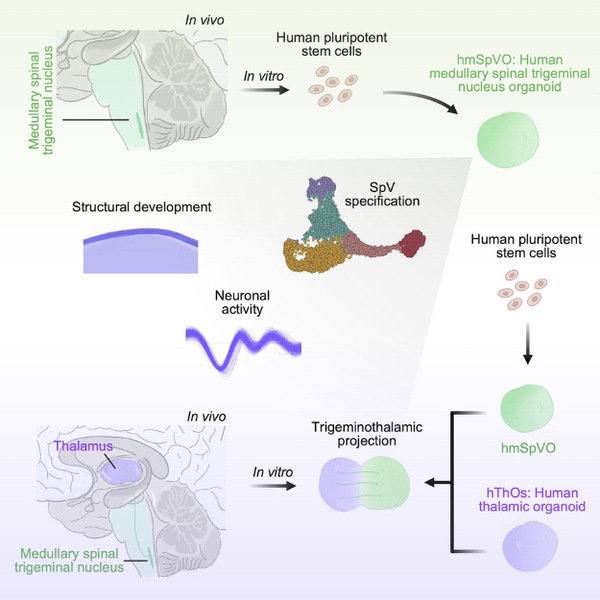
To model the connection between the SpV and the thalamus in vitro, researchers employed a fused culture of SpV organoids and thalamic organoids. The results showed SpV neurons exhibited a significantly stronger ability to project axons to the thalamic region compared to thalamic neurons projecting to the SpV region. The research team further analyzed the characteristics of projection establishment between different neural tissues through various fusion combinations, such as the fusion of SpV organoids with thalamic organoids, the fusion of SpV organoids with cortical organoids, and the fusion of thalamic organoids with cortical organoids. Projection patterns in fused brain organoids were consistent with the connection features noted between different brain areas in vivo. Additionally, through multiple research methods, including retrograde tracing, optogenetics, calcium imaging, and electrical stimulation and recording, the researchers further confirmed that connections between SpV cells and thalamic cells in the fused organoids were established.
This work marks the first successful construction of SpV-specific organoids and the establishment of an in vitro model for nucleus-associated neuronal connections. This model provides a valuable platform for studying human brain nucleus development, axonal targeting, and related pathological mechanisms. Graduate students Pang Wei and Zhu Jinkui from Professor Xiang’s group at the SLST, ShanghaiTech University, are co-first authors of this paper, with Xiang Yangfei as the corresponding author. ShanghaiTech University is the primary institution for this achievement.

