Hepatitis B virus (HBV) infection remains a severe global public health issue, with China bearing one of the highest burdens of HBV infection worldwide. It is estimated that over 80 million people in China are currently HBV carriers, with approximately 30 million cases diagnosed as chronic hepatitis B. The surface antigen of the HBV, known as HBsAg, is a critical protein responsible for the invasion, replication, and packaging of virus. It is also a key marker for diagnosis and prognosis. Understanding the 3D structure of HBsAg holds significant academic and practical implications for HBV research, as well as for the prevention and treatment of hepatitis B. However, its complex protein structure and diverse assembly patterns have long made it a challenging puzzle in structural biology and virology.
This structural study of HBsAg began twenty years ago, led by Rao Zihe, Distinguished Adjunct Professor at the Shanghai Institute for Advanced Immunochemical Studies (SIAIS) of ShanghaiTech University and Academician of the Chinese Academy of Sciences (CAS), alongside Hu Zhongyu, a Research Professor from the National Institutes for Food and Drug Control (NIFDC). Over these two decades, nearly twenty members of their joint research team have taken turns advancing the scientific breakthroughs in this field. Since joining ShanghaiTech in 2020, Wang Quan—formerly a student of Prof. Rao and now a Research Professor at SIAIS and Assistant Professor at the School of Life Science and Technology (SLST)—has been leading his team in studying the HBsAg structure. After nearly five years of dedicated work, based on a deep understanding of single-particle cryo-EM structure analysis and advancements in software, his team successfully resolved the 3D structure of HBsAg using their unique software and algorithms.
On September 12, this groundbreaking study on the structure of HBsAg was published in the journal Science under the title “Inherent Symmetry and Flexibility in Hepatitis B Virus Subviral Particles.” This collaborative research, led by Prof. Rao, Prof. Wang, and Dr. Hu, was conducted by a joint team from ShanghaiTech University, Tsinghua University, and the NIFDC.
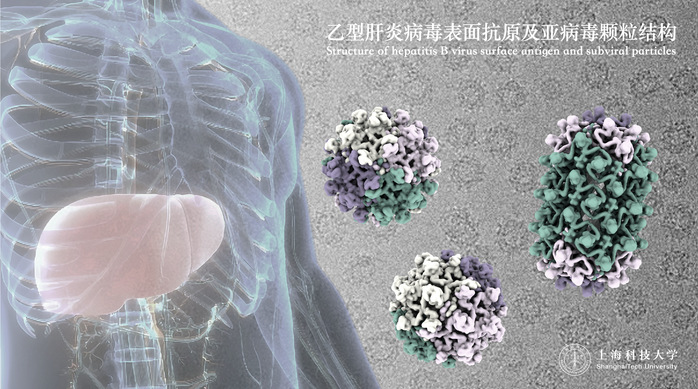
In this study, the research team successfully characterized the highly flexible surface proteins of HBV and their self-assembled patterns, revealing the molecular basis for the diverse oligomeric structures present on the surface of viral particles. Through observations and analyses of a typical trimeric and three different tetrameric local assembly patterns on subviral particles, the team elucidated the molecular mechanisms by which spherical particles extend to form longer tubular microfilament structures. They also detailed how these structures adapt to assemble with the internal nucleocapsid to form a complete virus.
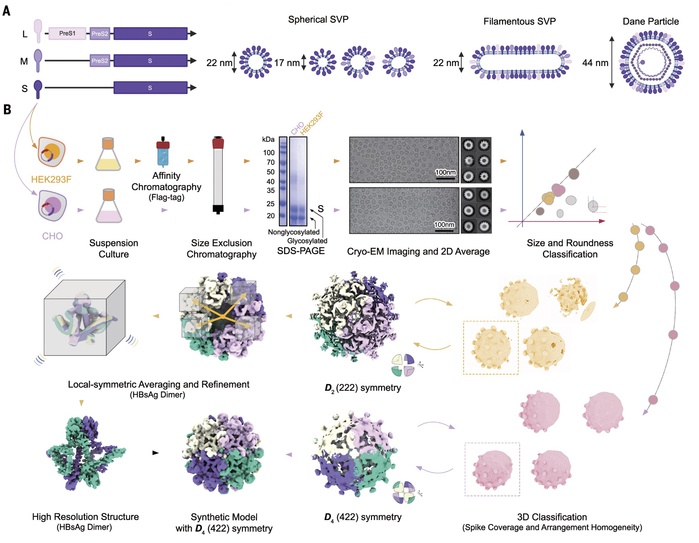
Main technical route and innovations
“This research not only solves a long-standing mystery in structural biology and virology but also provides critical insights for optimizing vaccines and understanding the structure-function relationships of neutralizing antibodies,” noted the paper’s reviewers. “In particular, this achievement is expected to accelerate the development of small molecules drugs that directly target viral surface proteins and envelope assembly, as well as protein degradation-targeted chimeras (PROTACs) that facilitate the breakdown of surface proteins. This breakthrough could unlock new strategies in combating HBV and significantly advance its treatment.”
Prof. Rao Zihe, Prof. Wang Quan, and Dr. Hu Zhongyu are the co-corresponding authors of the paper. Prof. Wang Quan, Prof. Wang Tao from Tsinghua University, Associate Professor Cao Lin from Nankai University, Dr. Fu Sheng and PhD student Mu An from the Institute of Biophysics, CAS, and PhD student Wang Peipei from SLST at ShanghaiTech University are the co-first authors. ShanghaiTech University is the primary affiliation.

