On November 12, 2024, Associate Professor Li Xiajun’s laboratory in the School of Life Science and Technology in ShanghaiTech University published a new study to further elucidate de novo and maintenance DNA methylation by DNA methyltransferases (DNMTs) in post-implantation embryos. This research was published in a paper titled “Regulation of de novo and maintenance DNA methylation by DNA methyltransferases in post-implantation embryos” in Journal of Biological Chemistry (JBC), a highly regarded journal with over 100 years of history, published by the American Society for Biochemistry and Molecular Biology (ASBMB).
As the major epigenetic modification of the genetic material DNA, DNA methylation is almost universally present in many living organisms, from bacteria to humans. It exerts a variety of essential functions in many important biological processes such as gene transcription, retroviral silencing and embryonic development. It also plays crucial roles in common human diseases including cancer and aging. There are three main DNMT proteins (DNMT1, DNMT3A and DNMT3B) in mice and humans that catalyze DNA methylation. It has been a long held belief that DNMT1 is the primary DNMT for the maintenance DNA methylation while DNMT3A and DNMT3B function in de novo DNA methylation, as described in some text books.
About two years ago, Prof. Li’s group published a paper in the journal iScience showing that all three DNMTs were required for maintaining DNA methylation across a wide range of genomic sequences in embryonic stem (ES) cells including repeats, genic and intergenic regions as well as some imprinting control regions (ICRs). These findings were consistent with a few previous studies implicating the roles of DNMT3A and DNMT3B in maintenance of DNA methylation in ES cells. However, they were in stark contrast to the more prevalent view claiming that DNMT1, but not DNMT3A and DNMT3B, is essential for maintenance DNA methylation while DNMT3A and DNMT3B function in de novo DNA methylation.
In this new study, Prof. Li’s group found DNMT3A and DNMT3B exerted maintenance and de novo DNA methylation in post-implantation mouse embryos. Together with DNMT1, they maintained DNA methylation at some pluripotent genes and lineage marker genes. Surprisingly, DNA methylation was increased at five ICRs after implantation, which was thought to be established only in the germline and then stably maintained in embryos after fertilization. Moreover, DNMT3A and DNMT3B maintained the newly acquired DNA methylation at two of these five ICRs. Intriguingly, DNMT3A and DNMT3B maintained pre-existing DNA methylation at four other ICRs, similar to what Prof. Li’s group found in ES cells previously that was published in the journal iScience on September 5, 2022. These results suggest that DNA methylation is more dynamic than originally thought during embryogenesis including the ICRs of the imprinted regions. DNMT3A and DNMT3B maintain large portions of newly acquired DNA methylation at various degrees across the genome in mouse embryos, together with DNMT1. Furthermore, they contribute to maintenance of pre-existing DNA methylation at a subset of ICRs as well as in the CpG islands (CGIs) and certain lineage marker genes. These findings may have implications for the important roles of DNMT proteins in embryonic development and human diseases.
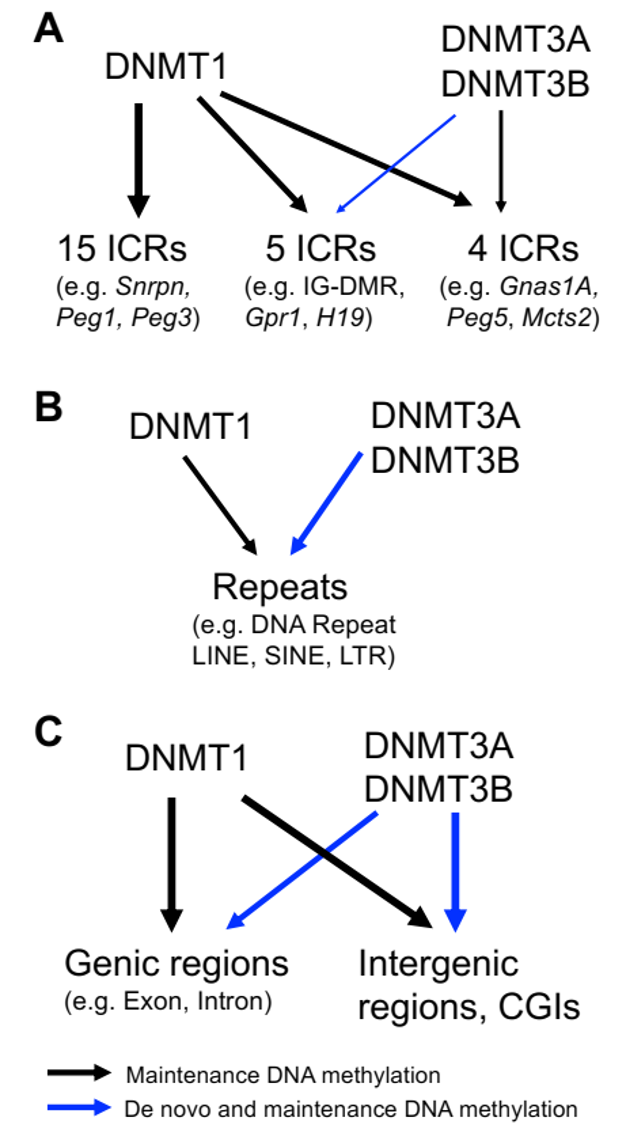
De novo and maintenance of DNA methylation by three DNMT proteins (DNMT1, DNMT3A and DNMT3B) in the post-implantation embryos. A) DNA methylation at the ICRs in the post-implantation embryos. B) DNA methylation at the repeats such as LINE, SINE and LTR in the post-implantation embryos. C) DNA methylation in the genic and intergenic regions in the post-implantation embryos including exons, introns and CGIs.
*This article is provided by Prof. Li Xiajun

