Aromatic hydrocarbons are essential raw materials in chemical production, finding widespread application in the synthesis of plastics, dyes, fragrances, pharmaceuticals, and synthetic rubber. Technologies that convert non-petroleum carbon sources, such as syngas and methanol, into aromatics within molecular sieves have garnered significant interest for their potential to reduce reliance on petroleum. Past studies have established that during aromatic synthesis, olefin-induced hydrogen-transfer (OHT) reactions are inevitable, producing substantial alkane byproducts and thereby limiting aromatic selectivity. Recent research has shown that introducing carbon monoxide (CO) into the reaction suppresses alkane selectivity while markedly enhancing aromatic selectivity. However, a microscopic understanding of this phenomenon has remained elusive.
Recently, Associate Professor Yang Bo’s research group at the School of Physical Science and Technology (SPST) at ShanghaiTech University achieved a significant breakthrough in their study of HZSM-5 zeolite-catalyzed indirect methane conversion to aromatics. Using ab initio molecular dynamics (AIMD) simulations coupled with free energy sampling techniques, including metadynamics and slow-growth methods, the team uncovered a novel ketene/acetyl-mediated hydrogen-transfer mechanism that outperforms olefin-induced pathways. This discovery holds substantial promise for enhancing aromatic selectivity. The findings were published in the international journal Nature Communications.
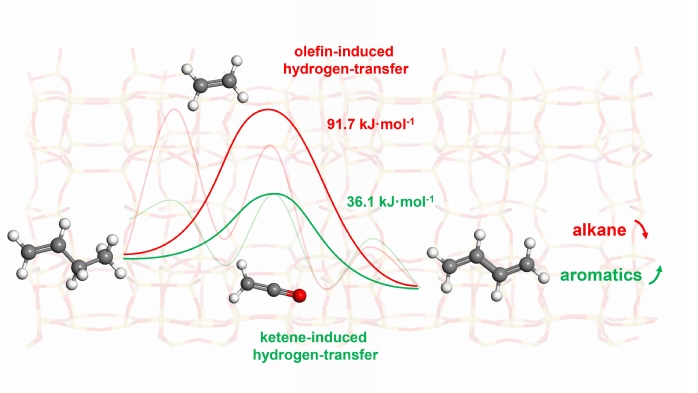
Advantages and roles of ketene/acetyl-induced hydrogen-transfer reactions in CO-coupled aromatic synthesis.
In this study, the researchers constructed a general mechanism applicable to a series of CO-coupled aromatic synthesis reactions, offering a detailed microscopic perspective for optimizing zeolite catalyst design.
Drawing on experimental observations of surface acetyl species and the pronounced reduction in alkane selectivity under a CO atmosphere, the researchers proposed a ketene-induced hydrogen-transfer mechanism, a new mechanism that may compete with OHT. Through AIMD simulations and free energy sampling including metadynamics and slow-growth, they modeled the free energy surfaces (FES) for reactions where ethylene and ketene/acetyl cations serve as hydride acceptors in the conversion of butylene to butadiene. The results revealed that the ketene/acetyl cation-mediated pathway has an effective free energy barrier of just 36.1 kJ/mol, significantly lower than the 91.7 kJ/mol barrier for the ethylene-induced OHT route. This indicates that ketene/acetyl cations are more effective hydride receptors than olefins, enabling a route that increases olefin unsaturation without generating additional alkanes. Moreover, the resulting butadiene and acetaldehyde can further participate in cyclization reactions, boosting aromatic formation.
The first author of the paper is Guo Zhichao, a fourth-year master’s student at ShanghaiTech, with Prof. Yang Bo as the corresponding author. ShanghaiTech is the sole affiliation.

