Natural gas, a clean and abundant fossil fuel, is primarily methane, sourced from conventional reservoirs and unconventional deposits like coal beds, tight formations, shale, and marine sediments. These diverse sources collectively underpin the global natural gas supply. Yet, methane’s high chemical stability—stemming from its symmetry, strong C-H bonds, and low polarizability—limits its use as a chemical feedstock. Traditional methods like steam methane reforming are associated with high emissions and energy consumption. In comparison, methane pyrolysis has significant environmental and economic potential, converting methane into high-value hydrogen and carbon materials without generating CO₂ greenhouse gases. However, its commercial adoption has been hindered by catalyst instability and harsh process conditions.
A team of scientists led by Assistant Professor Guan Xiaofei from the School of Physical Science and Technology (SPST) at ShanghaiTech University has developed a novel method: methane pyrolysis via molten sodium chloride (NaCl) electrolysis. This approach effectively decomposes methane at moderate temperatures (400–660°C) into high-value chemical products such as hydrogen, ethylene, and carbon materials. The research was recently published in the prestigious journal ACS Catalysis and featured on its Front Cover, marking a significant leap forward.

The achievement was featured as the Front Cover of ACS Catalysis.
The researchers integrated electrochemical and thermochemical processes by leveraging the well-established molten salt electrolysis technology from the metallurgical industry. In this process, chloride ions are oxidized at the anode into chlorine gas, while sodium ions are reduced to metallic sodium at the cathode. A small amount of sodium dissolves in the molten electrolyte, producing solvated electrons. When methane is introduced into the molten electrolyzer, it reacts rapidly with chlorine at the anode, forming chlorinated hydrocarbons and hydrogen chloride. As the gases rise, these intermediates react with metallic sodium or solvated electrons at the cathode, yielding hydrogen, ethylene, and carbon materials. The chlorine ions are then recycled back into the molten electrolyte, forming a closed-loop system.
Compared to traditional high-temperature methane pyrolysis, this method operates under milder conditions and exhibits stable performance. The resulting carbon, easily separated and purified, has potential applications as an anode material in batteries. This innovative approach, which involves the formation and reduction of halogenated intermediates, represents a versatile and efficient technology applicable not only to methane decomposition but also to the conversion of other hydrocarbons.
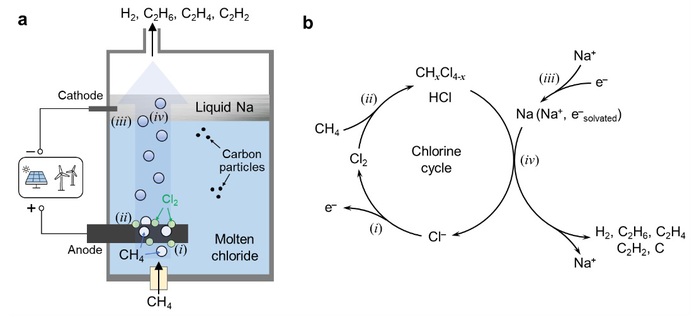
Working principle of methane decomposition enabled by molten metal chloride electrolysis.
In laboratory-scale experiments using a ternary molten salt system composed of lithium chloride, sodium chloride, and potassium chloride, a methane conversion rate of approximately 30% was achieved at 550°C, with hydrogen selectivity at 70% and ethylene at 5.3%. The carbon product, with a purity of about 99.66%, can be easily separated from the salt via water washing. Increasing the temperature within the range of 400–550°C enhances the selectivity of hydrogen and ethylene, while increasing the current boosts the rate of NaCl electrolysis, producing more sodium and chlorine gas and thus improving methane conversion and hydrogen selectivity. Alkali metal bromides and alkaline earth metal chlorides can also be used as molten electrolytes for methane decomposition.
Zhang Xu, a second-year PhD student at SPST, is the first author of the paper, with Prof. Guan Xiaofei as the corresponding author. Co-authors include Dr. Liu Jian, Li Wenda, Zhou Jiayin, Associate Professor Yang Bo, and Assistant Professor Xu Chao, all from the SPST. ShanghaiTech University is the sole affiliation.
*This article is provided by Prof. Guan Xiaofei

