Recently, a team led by Assistant Professor Huang Huan-Ming from the School of Physical Science and Technology at ShanghaiTech University published a groundbreaking achievement in the top-tier journal Nature Communications. They developed a new method that uses an organophotocatalyst and a chromium catalyst to transform three simple ingredients into complex “chiral” molecules in a single step under visible light condition. This technique is not only efficient but also precisely controls molecular structures, making it a “magic combo” in chemical reactions.
A “puzzle challenge” in chemistry
In chemistry, scientists often need to piece together simple molecules into complex ones, much like solving a puzzle. But when the puzzle pieces must fit perfectly in shape and direction (known as “chirality”), it gets tricky. Chiral molecules are like left and right hands—mirror images that don’t overlap. The key lies in a “chiral center,” a special point in the molecule, usually a carbon atom, surrounded by four different “partners,” acting as a “crossroad” that sets the direction. This property gives molecules a “left-hand” or “right-hand” form, and in drugs, often only one form works. Traditional reactions are either tedious or hard to control, especially with “radicals”—lively but unruly chemical intermediates.
A “dance” of light and chromium
Prof. Huang’s team took a fresh approach with dual photoredox/chromium catalysis technique. They shine blue light on the reaction mixture, triggering a simple aromatic ring to produce radicals, which are then tamed by a chromium catalyst to form the target molecule. Specifically, they mix three ingredients—aromatic compounds, 1,3-dienes, and aldehydes (some derived from drug molecules). With light’s help, the aromatic compound breaks a C-H bond to form a radical cation, quickly reacting with the 1,3-diene to create an intermediate. The chromium catalyst then guides this intermediate to bond with the aldehyde, producing a molecule with two chiral centers in one go—think of it as controlling two “crossroads” at once to lock in a specific “left-hand” or “right-hand” shape. The yield reaches up to 90%, with near-perfect chirality control (enantioselectivity).
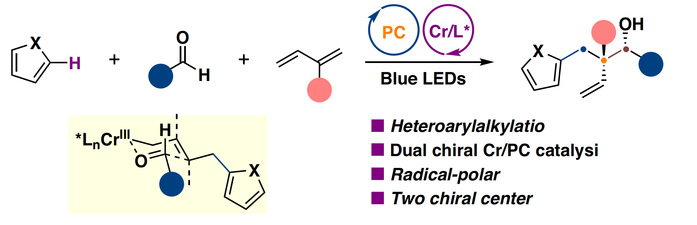
This method is like a well-choreographed “dance”: light kicks things off, and chromium steers the direction, ensuring a complex, precise product. The team also found the reaction is “forgiving,” working well with everyday chemicals and drug-related molecules alike.
From lab to pharmacy
This technique shines by rapidly creating “homoallylic alcohols”—building blocks common in natural products and drug development. With chiral centers, molecules gain a “steering wheel,” splitting into two mirror forms. In drugs, the “left hand” might heal, while the “right hand” could be useless or harmful—this method ensures the “right hand” is made. Some aldehydes used come from drug derivatives, hinting at direct pharmaceutical applications. Even better, the reaction runs under mild conditions (room temperature), no need for extreme heat or pressure, making it simple and scalable.
Early experiments uncovered the “secret”: radicals are the stars, while the chromium catalyst shapes the chiral centers via a special transition state (called the “Zimmerman-Traxler” model). This “radical-to-polar crossover” mechanism makes the reaction fast and accurate, solving a puzzle traditional method struggles with.
The paper’s first author is postdoc researcher Tang Si-Yuan, with Assistant Professor Huang Huan-Ming as the corresponding author. “We hope this technique sparks new ideas for drug synthesis and material development,” Huang said. The work was conducted entirely at ShanghaiTech. This work not only showcases chemical innovation but also sets a model for combining light and base metal catalyst.

