A new study published in Cell has uncovered how a molecule called LAG3 functions as a brake to suppress the immune system. This discovery helps explain how cancer avoids immune attacks and may guide doctors to better treatment options. Led by Associate Professor Wang Haopeng from the School of Life Science and Technology (SLST) at ShanghaiTech University, this research offers a fresh perspective for improving cancer immunotherapy.
LAG3: the immune system’s “brake pedal”
T cells are the body’s defenders, tasked with finding and destroying viruses and cancer cells. However, the immune checkpoint LAG3 acts like a “brake pedal” on T cells, stopping them from working. Cancer takes advantage of LAG3 to inhibit T cell activity, allowing tumors to grow unchecked. Following the landmark approvals of PD-1 and CTLA-4 inhibitors, the U.S. FDA granted approval in 2022 to LAG3-targeted therapeutics, establishing them as the third class of immune checkpoint inhibitors to enter clinical practice. This milestone represents a crucial expansion of the cancer immunotherapy arsenal, but until now, scientists did not fully understand how LAG3 suppresses T cell anti-tumor immunity. This fundamental question has long stood as a pivotal scientific challenge perplexing the field for decades. This study provides the answer.
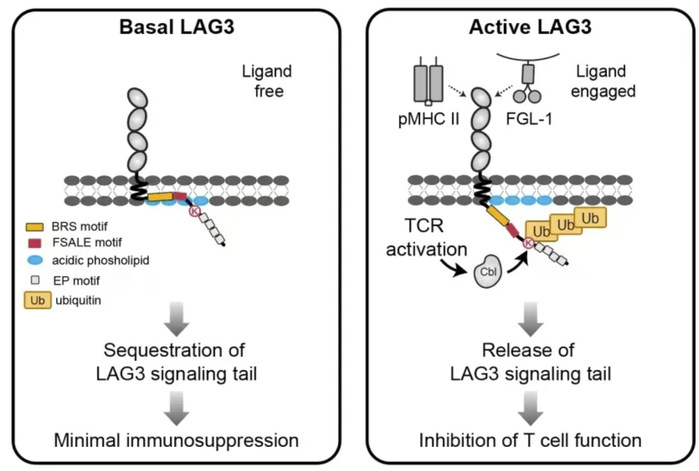
Diagram of the activation mechanism of the immune checkpoint receptor LAG3
Key finding 1: Ubiquitination as a LAG3-activating molecular switch.
The research shows that when LAG3 meets specific ligands—like major histocompatibility complex class II (MHC class II) molecules or membrane-bound fibrinogen-like protein 1 (FGL1)—it quickly gets a small tag (Ubiquitin) at a specific spot (K498 site) during T cell activation. This process starts within 2 minutes and peaks at 5 minutes. Once tagged, LAG3 becomes more effective at putting the brakes on T cells, keeping them weaker and less able to attack tumors.
Key finding 2: Ubiquitination orchestrates a “sequestration-to-exposure” dynamic regulatory mechanism.
When LAG3 is off, its signal motif inside the cell stays buried into the cell membrane like a locked box—can’t send signals. But when it connects to its partner molecule, a ubiquitin tag pops open the box. This releases the signal motif, flipping on LAG3’s “stop” button that weakens T cells’ attack power.
Key finding 3: Cbl family mediates LAG3 ubiquitination.
The study identified E3 ubiquitin ligases called the Cbl family, which assist LAG3 in its role.The Cbl family act like “brake installers” for LAG3, adding the ubiquitin tag to LAG3, making it better at weakening T cells. With Cbl’s help, LAG3 protects tumors by keeping T cells dormant. In experiments, when scientists removed the Cbl family, LAG3 stopped working properly, and T cells became active again. This suggests that LAG3-Cbl partnership normally shields tumors by blocking T cells.
Why this matters
New cancer treatments: Understanding how LAG3 works could lead to methods to stop LAG3, enabling T cells to attack tumors.
Choosing the right patients: When LAG3 and Cbl work together strongly, T cells are heavily suppressed. Doctors can test for high levels of LAG3 and CBL proteins to identify patients who will benefit most from drugs that block LAG3, improving treatment success.
Scientific insight: Previously, ubiquitin tagging was thought to only target proteins for degradation, but this study shows it can also activate them, offering a new view of immune regulation.
Researchers stated: “We knew LAG3 could stop the immune system, but we didn’t know how it switched on. This finding is like a key that could help more cancer patients benefit from better therapies.”
The research team plans to explore ways to block ubiquitination, preventing LAG3 from putting the brakes on T cells, or to use this discovery to develop smarter treatments. This could bring new hope to the fight against cancer.
Postdoctoral researcher Jiang Yong at SLST, Dr. Dai Anran (graduated in 2025), and third-year PhD student Huang Yuwei are three of the co-first authors. Prof. Wang Haopeng is the last corresponding author. ShanghaiTech University is the primary affiliation.

