Heterochromatin is a tightly packed form of DNA essential for maintaining genome stability, regulating gene expression, and ensuring proper chromosome segregation during cell division. Despite its importance, the molecular mechanisms underlying heterochromatin formation remain incompletely understood. In eukaryotic cells, heterochromatin is marked by specific histone modifications, such as methylation of histone H3 at lysine 9 (H3K9me), recruiting proteins like HP1 to establish a transcriptionally silent state. Dysregulation of heterochromatin assembly is linked to genomic instability, developmental disorders, and cancer.
While the RNA interference (RNAi) pathway has been extensively studied in heterochromatin formation, recent evidence suggests that RNA-binding proteins and phase separation may also play crucial roles. However, how these processes cooperate to assemble heterochromatin, particularly at repetitive DNA regions like centromeres, remains unclear. Additionally, differences in heterochromatin regulation across species complicate efforts to establish conserved mechanisms.
Recently, the team led by Assistant Professor Tomoyasu Sugiyama from the School of Life Science and Technology (SLST) at ShanghaiTech University uncovers a pivotal role for the nuclear poly(A)-binding protein Pab2 (PABPN1 in humans) in heterochromatin assembly. The study was published in a paper titled “The nuclear poly(A)-binding protein Pab2/PABPN1 promotes heterochromatin assembly through the formation of Pab2 nuclear condensates” in the journal PLOS Genetics.
In the study, using the fission yeast Schizosaccharomyces japonicus as a model, the team discovered that: (1) Pab2 is essential for H3K9 methylation, and deletion of Pab2 significantly reduces H3K9me2/3 levels at centromeres; (2) Phase separation drives Pab2 function—Pab2 forms nuclear condensates through its RNA-binding domain (RRM) and intrinsically disordered region (IDR), selectively recruiting heterochromatin factors like the H3K9 methyltransferase Clr4 and the remodeler Mit1; (3) Species-specific regulatory mechanisms—Unlike in S. pombe, Pab2 in S. japonicus is indispensable for growth and meiosis, highlighting evolutionary divergence in heterochromatin regulation; (4) A network of RNA-binding partners—Pab2 interacts with proteins Red5, Rmn1, and Ppn1 to stabilize heterochromatin, with disruptions in these interactions leading to defective silencing.
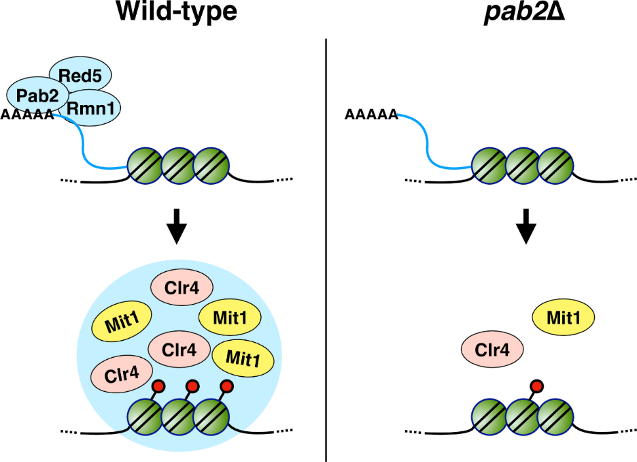
A model for Pab2-mediated heterochromatin assembly in S. japonicus.
In conclusion, this study not only reveals a novel mechanism by which Pab2 promotes heterochromatin assembly through phase-separated condensates, facilitating Clr4 and Mit1 recruitment to centromeres, but also advances our understanding of RNA-binding proteins and phase separation in heterochromatin formation. This discovery provides fresh insights into the molecular mechanisms underlying heterochromatin establishment and may offer new therapeutic avenues for related diseases. Future research will further investigate the dynamic properties of Pab2 condensates and their relationship with heterochromatin domains, as well as examine the role of polyadenylation in heterochromatin assembly and investigate the functional conservation of Pab2 across different species.
Liu Ziyue, a postdoctoral researcher in Tomoyasu Sugiyama’s group, is the first author of this paper. Tomoyasu Sugiyama is the corresponding author. ShanghaiTech University is the first affiliation and corresponding institution for this work.
*This article is provided by Prof. Tomoyasu Sugiyama

