Imagine a world where a child born deaf can hear their parents’ voices for the first time. This vision is closer to reality thanks to a groundbreaking discovery by researchers at iHuman Institute, ShanghaiTech University. Led by Associate Professor Zhong Guisheng, the team has developed a technology called ARBITER (AAV Reporter-Based in vivo Transcriptional Enhancer Reconstruction), which has successfully restored hearing in deaf mice by precisely targeting outer hair cells in the ear. Published on April 21in Neuron under the title “Deciphering Enhancers of Hearing Loss Genes for Efficient and Targeted Gene Therapy of Hereditary Deafness,” this study marks a significant leap forward in treating hereditary deafness, a condition affecting over 400 million people worldwide, including 34 million children.
Hereditary deafness often stems from mutations in genes like Slc26a5, which encodes a protein essential for outer hair cells—the ear’s “sound amplifiers.” When Slc26a5 mutates, these cells fail to amplify sound, leading to profound hearing loss. Traditional gene therapies using adeno-associated viruses (AAVs) to deliver corrected genes face challenges: the virus can mistakenly target other types of cells, causing side effects, or require high doses that increase risks and costs. ARBITER overcomes these hurdles by identifying and optimizing “super keys”—highly specific regulatory sequences that ensure the Slc26a5 gene is expressed only in outer hair cells.
Think of the ear as a bustling factory where outer hair cells are technicians relying on a Slc26a5 “manual” to amplify sound. A mutation scrambles the manual, shutting down the factory. ARBITER acts like a master locksmith, crafting a super key that unlocks the correct manual exclusively for these technicians. In tests on deaf mice and tree shrews, AAVs equipped with ARBITER’s super key delivered the Slc26a5 gene to outer hair cells, restoring their function and enabling the animals to hear again, as confirmed by auditory tests.
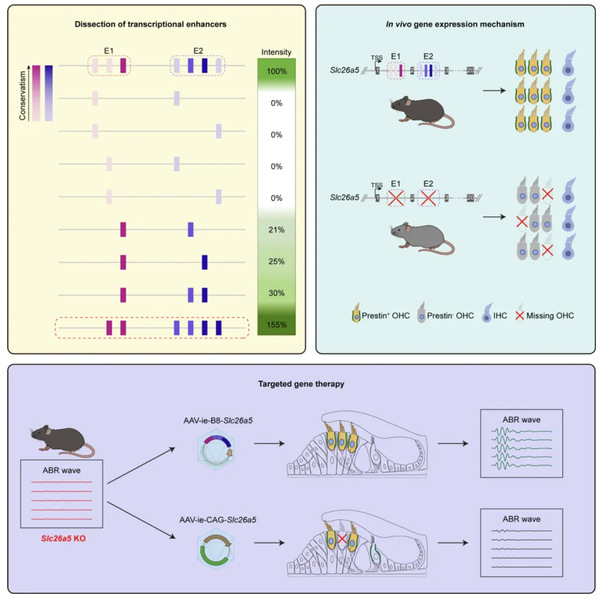
What sets ARBITER apart is its versatility. Beyond Slc26a5, it can identify regulatory keys for other deafness genes, such as Tmc1 or Gjb2, paving the way for tailored therapies for various forms of hereditary deafness. This technology also deepens our understanding of how genes are regulated in the cochlea, offering insights into ear development and potential applications for other genetic disorders.
“This breakthrough is a game-changer for hereditary deafness research,” said Prof. Zhong Guisheng. “ARBITER not only restores hearing in animal models but also sets a foundation for safer, more precise gene therapies that could one day transform lives.”
The study’s co-first authors, Research Associate Professor Zhao Simeng from iHuman Institute and PhD students Yang Qiuxiang and Yu Zehua from the School of Life Science and Technology, played pivotal roles in developing ARBITER. Prof. Zhong and Dr. Zhao are the co-corresponding authors, with ShanghaiTech University as the first affiliation. The team is now exploring ARBITER’s potential to target other cochlear cell types and complex genetic diseases, bringing hope to millions affected by hearing loss and beyond.

