On June 24, a research team led by Assistant Professor Hua Tian and Professor Liu Zhi-Jie from the iHuman Institute and the School of Life Science and Technology at ShanghaiTech University published a study in Nature via Accelerated Article Preview (AAP). Titled “Structural and functional characterization of human sweet taste receptor,” the study reports the cryo-EM structures of the human sweet taste receptor in both ligand-free (apo) and sucralose-bound states. It systematically reveals the receptor’s unique asymmetric heterodimeric architecture and ligand recognition mechanism, representing a significant step forward in deciphering the molecular basis of sweet taste perception.
Sweetness is one of the five basic human taste modalities and plays an essential role in energy intake and nutritional balance. Evolutionarily, the ability to detect sweetness helped early humans identify sugar-rich, energy-dense foods. Modern research has shown that sweet taste not only activates the brain’s reward system to produce pleasurable sensations, but also participates in blood glucose regulation and energy metabolism. However, the overconsumption of sugars in today’s society has contributed to a global rise in obesity, diabetes, and related health issues. Therefore, understanding the molecular mechanisms of sweet taste receptors and developing safer sugar substitutes have become global priorities. Notably, sweet taste receptors are expressed not only in taste bud cells but also in the gut, pancreas, and other tissues, making them promising therapeutic targets for metabolic disorders.
Similar to receptors for sour, bitter, salty, and umami tastes, sweet perception begins with the activation of specialized taste cells in the tongue. The human sweet taste receptor is a heterodimer composed of TAS1R2 and TAS1R3 subunits, members of the class C G protein-coupled receptor (GPCR) family. It can recognize a wide variety of sweet compounds, including natural sugars (e.g., sucrose), artificial sweeteners (e.g., sucralose, aspartame, neotame), and sweet-tasting proteins (e.g., thaumatin) (Figure 1). However, due to the receptor’s low expression yield and conformational instability in heterologous systems, structural determination has been extremely challenging. As a result, detailed 3D structural insights and molecular mechanisms underlying ligand recognition have remained elusive for years, presenting a major hurdle in the field.
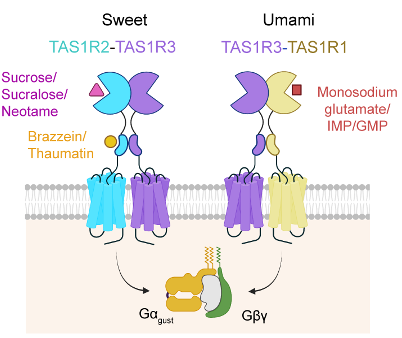
Figure 1: Schematic diagram of gustducin-mediated signal transduction pathways activated by various ligands through the sweet taste receptor (TAS1R2-TAS1R3) and the umami taste receptor (TAS1R1-TAS1R3).
After sustained effort and optimization, the team overcame these challenges by incorporating various fluorescent protein tags to improve receptor stability and expression. Using single-particle cryo-electron microscopy, they successfully resolved the structures of the TAS1R2-TAS1R3 heterodimer in apo and sucralose-bound states (Figure 2). The overall architecture reveals that both subunits contain canonical class C GPCR domains: a Venus flytrap domain (VFTD), a cysteine-rich domain (CRD), and a seven-transmembrane domain (TMD). Interestingly, the receptor adopts an unexpected asymmetric dimer conformation in both states, with TAS1R2-VFTD tending to a “closed” conformation, while TAS1R3-VFTD remains “open.” Cryo-EM density further shows that sucralose binds specifically within the VFTD of TAS1R2, interacting with key residues (e.g., D142, E302, Y103) through hydrogen bonds, polar, and hydrophobic interactions (Figure 3a–b).
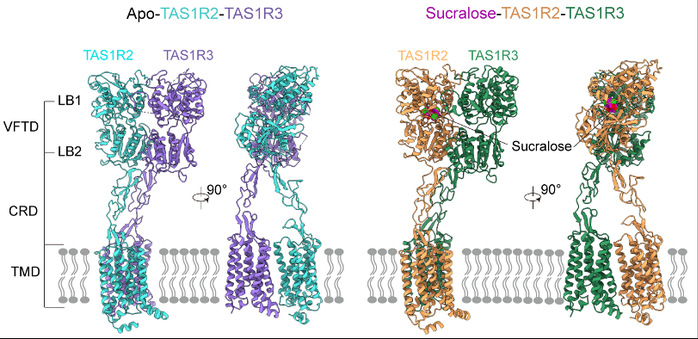
Figure 2: Three dimensional structures of the sweet taste receptor TAS1R2-TAS1R3 in the apo state (left) and sucralose-bound state (right).
To validate these findings, the team developed a sensitive pharmacological assay at the cellular level (Figure 3c), enabling robust detection of weakly active natural sweeteners. Using this assay, they confirmed the functional importance of residues involved in sucralose binding and further explored the binding modes of sucrose and neotame via molecular docking and functional studies. Despite sharing the same binding pocket within TAS1R2, each sweetener interacts differently with the receptor, revealing distinct molecular mechanisms that explain variations in perceived sweetness.
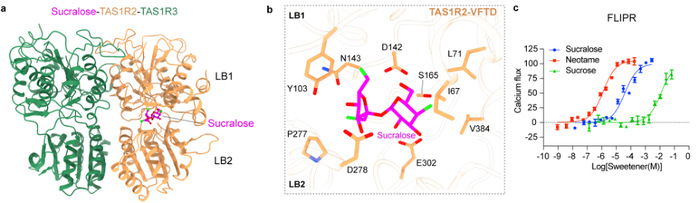
Figure 3: Binding mode of sucralose in the sweet taste receptor. (a) Sucralose binds to the VFTD of TAS1R2, while the VFTD of TAS1R3 remains in an open conformation; (b) Binding pocket of sucralose and key residues involved in receptor interaction; (c) Relative activation potency of sucralose, neotame, and sucrose on the sweet taste receptor measured using the FLIPR® fluorescence microplate reader.
Structural comparison of the apo and sucralose-bound states revealed that ligand-induced conformational changes in the sweet receptor are relatively subtle compared to the large-scale rearrangements typically observed in other class C GPCRs. Specifically, conformational shifts in TAS1R2-VFTD are transmitted to the CRD via a disulfide bond (C233–C513), leading to CRD bending and a slight TMD rotation. Moreover, a five-residue loop within TAS1R3’s CRD inserts between the two VFTDs, facilitating cooperative conformational changes between subunits. These unique features, not observed in other class C GPCRs, suggest a novel activation mechanism for the sweet taste receptor. Additionally, AlphaFold3 predictions of the receptor-G protein complex suggest that TAS1R2 may play a dominant role in G protein coupling and signal transduction.
This study provides a comprehensive structural and functional framework for understanding how the human sweet taste receptor recognizes and responds to different sweeteners. It offers critical insights for the rational design of next-generation sweeteners and for drug discovery targeting metabolic diseases.
The co-first authors of the paper are: 2024 PhD student Shi Zongjun, Postdoctoral Fellow Xu Weixiu, Research Associate Professor Wu Lijie, and 2023 PhD student Yue Xiaolei. Hua Tian and Liu Zhi-Jie are the co-corresponding authors, with ShanghaiTech University as the first affiliation.
*This article is provided by Prof. Hua Tian

